Always refer to the main Pearson general guidelines and science guidance when writing alternative text for images.
When describing Chemistry images, keep in mind the following key aspects and examples. Not all guidance will apply to all images.
- Identify the content of the image by describing what the image shows (i.e., chemical structures, reactions, symbols, equations, etc.)
- Avoid using complex language. The description should mirror language in the main text.
- Keep the alt text brief. Identify the key elements of the image and focus the description on those elements.
- If the chemistry image is part of a larger body of art work, it may be helpful to provide some context in the alt text (e.g., a molecular model appears with a diagram of a biochemical process).
Examples
Reminder: The Mastering authoring platform has a title field and alt text field but does not have the functionality for a long description. Alternative text descriptions in Mastering may have more than 255 characters. The eText 2 authoring platform has the functionality for alt text and a long description. For more information about the different authoring systems, see Platform Authoring Information.
Example 1
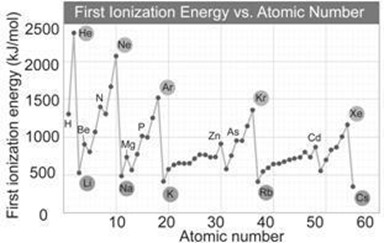
Alt Text
A graph plots first ionization energy, in kilojoules per mole, versus atomic number. In the graph, alkali metals are at valleys, and noble gases are at peaks. The graph includes the following points for different elements. All data are approximate. Hydrogen, (1, 1300). Helium, (2, 2450). Lithium, (3, 505). Beryllium, (4, 900). Nitrogen, (7, 1400). Neon, (10, 2150). Sodium, (11, 495). Magnesium, (12, 750). Phosphorus, (15, 1005). Argon, (18, 1505). Potassium, (19, 400). Zinc, (30, 900). Arsenic, (33, 950). Krypton, (36, 1350). Rubidium, (37, 400). Cadmium, (48, 900). Xenon, (54, 1200). Cesium, (55, 350).
Example 2
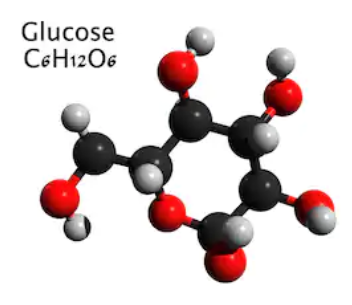
Alt Text
Ball-and-stick model is shown for glucose. Glucose (C 6 H 12 O 6) is a six-membered ring, with carbon at five vertices and oxygen at the other. Starting at the carbon adjacent to the oxygen atom and moving clockwise: carbon 1 has an H atom up and O H group down, carbon 2 has an H atom up and O H group down, carbon 3 has an H atom up and O H group down, carbon 4 has an O H group up and H atom down, carbon 5 has an H atom up and C H 2 O H group down.
Example 3
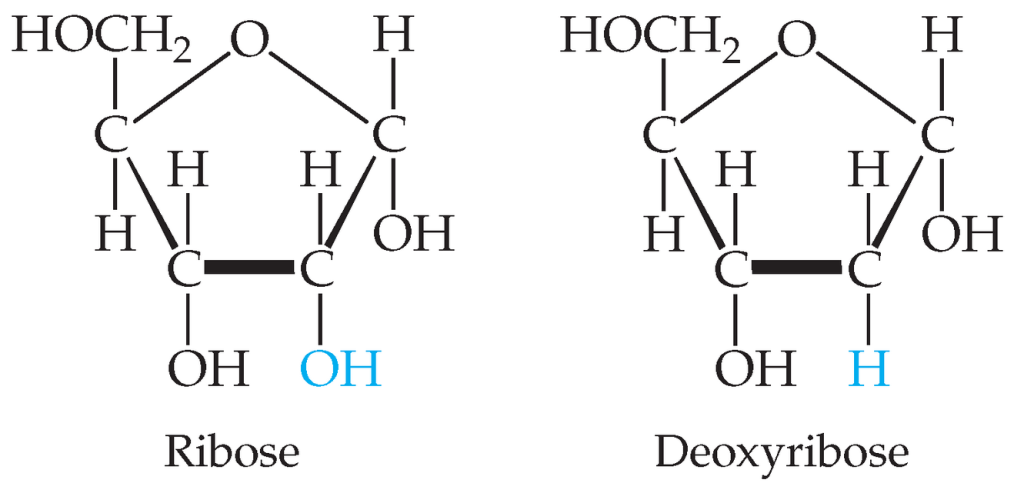
Alt Text
Ribose and deoxyribose are nearly identical, with the only difference being a single oxygen atom. Both ribose and deoxyribose form a pentagon, arranged with its point oriented up. That top point is oxygen; the other four vertices are carbons. The upper right vertex is single bonded below to O H and above to H. The lower right vertex is where the only difference between ribose and deoxyribose is found. Ribose is single bonded below to O H while deoxyribose is single bonded below only to H. Both molecules are single bonded above to H. The lower left vertex is single bonded below to O H and above to H, and the upper left vertex is single bonded below to H and above to C H 2 O H .
Example 4
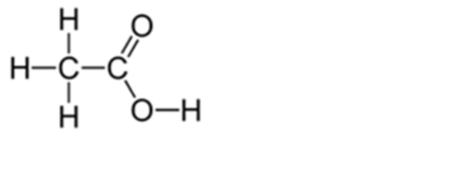
Alt Text
H, single bond, C, single bond C. C 1 is single bonded to H above and H below. C 2 is double bonded to O on the upper right and single bonded to O on the lower right. The second O atom is single bonded to H on the right.
Example 5
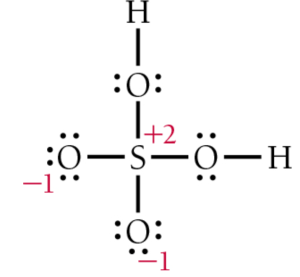
Alt Text
Lewis structure of H 2 S O 4 with charges. A central S is single bonded to 4 O, 2 with 3 lone pairs, and 2 with 2 lone pairs. Each O with 2 lone pairs is single bonded to an H. Charges are as follows. S, plus 2. Each O with 3 lone pairs, minus 1.
Example 6
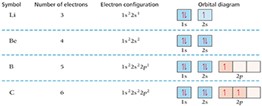
Alt Text
A table provides the symbol, number of electrons, electron configuration, and orbital diagram for 4 elements, L i, B e, B, and C.
Long Description
(Must be marked up in HTML)
| Symbol | Number of electrons | Electron configuration | Orbital diagram |
|---|---|---|---|
| L i | 3 | 1 s 2, 2 s 1 | The orbital diagram has boxes filled as follows. 1 s, filled, with 2 arrows. 2 s, half filled, with 1 arrow. |
| B e | 4 | 1 s 2, 2 s 2 | The orbital diagram has boxes filled as follows. 1 s, filled, with 2 arrows. 2 s, filled, with 2 arrows. |
| B | 5 | 1 s 2, 2 s 2, 2 p 1 | The orbital diagram has boxes filled as follows. 1s, filled, with 2 arrows. 2 s, filled, with 2 arrows. 2 p has three boxes. The first box is half-filled, with 1 arrow. The second and third boxes are empty. |
| C | 6 | 1 s 2, 2 s 2, 2 p 2 | The orbital diagram has boxes filled as follows. 1s, filled, with 2 arrows. 2 s, filled, with 2 arrows. 2 p has three boxes. The first two boxes are each half-filled, with 1 arrow. The third box is empty. |
Example 7
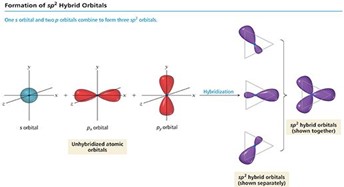
Alt Text
The formation of s p 2 hybrid orbitals.
Long Description
(Must be marked up in HTML)
One s orbital and 2 p orbitals combine to form 3 s p 2 orbitals. Each unhybridized atomic orbital is shown on an x y z coordinate plane, as follows.
- The s orbital is represented by a sphere centered at the origin.
- The p sub x orbital is represented by 2 lobes on the x axis, with thinner ends centered at the origin.
- The p sub yorbital is represented by 2 lobes on the y axis, with thinner ends centered at the origin.
Following hybridization, the s p 2 hybrid orbitals extend toward 3 points of a triangle.
Example 8
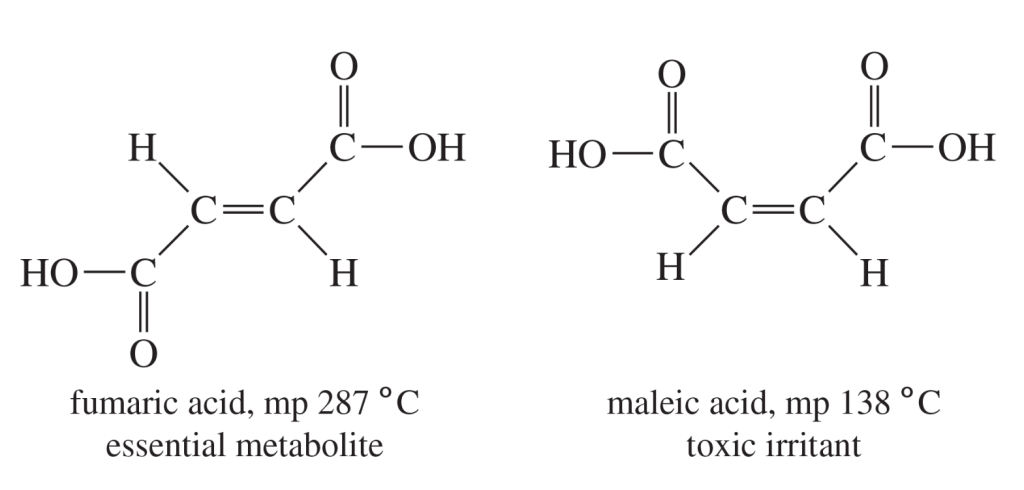
Alt Text
Structures of fumaric acid and maleic acid and their melting points and properties are shown.
Long Description
(Must be marked up in HTML)
Fumaric acid and maleic acid have the following structure: H O single bond C single bond C double bond C single bond C single bond O H. C 1 has a double bond to O. C 2 has a single bond to H. C 3 has a single bond to H. C 4 has a double bond to O.
- In fumaric acid, C 1 and C 4 are located on the opposite sides of the double bond. Fumaric acid has a melting point of 287 degree Celsius. It is an essential metabolite.
- In maleic acid, C 1 and C 4 are on the same side of the double bond. Maleic acid has a melting point of 138 degree Celsius. It is a toxic irritant.
Example 9
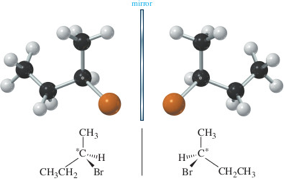
Alt Text
A ball-and-stick model, a structure of 2-bromobutane, and their mirror images are shown.
Long Description
(Must be marked up in HTML)
- A ball-and-stick model shows four successively connected black balls. The first (from left to right) black ball has three white balls single bonded. The second black ball has two white balls single bonded. The third black ball has a white ball single bonded and an orange ball single bonded. The fourth black ball has three white balls single-bonded.
- The mirror image of the ball-and-stick model shows four successively connected black balls. The first (from left to right) black ball has three white balls single bonded. The second black ball has a white ball single bonded and an orange ball single bonded. The third black ball has two white balls single bonded. The fourth black ball has three white balls single-bonded.
- The structure shows a central C atom. It has a single bond to C H sub 3 at the top position, a single bond to C H sub 2 C H sub 3 at the bottom-left position, a dashed wedge to H at the right position, and a solid wedge to B r at the bottom-right position. There is an asterisk near the central C.
- The structure of the mirror image shows a central C atom. It has a single bond to C H sub 3 at the top position, a single bond to C H sub 2 C H sub 3 to the bottom-right position, a dashed wedge to H at the left position, and a solid wedge to B r at the bottom-left position. There is an asterisk near the central C.
Example 10
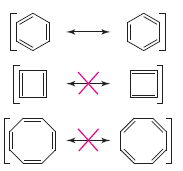
Alt Text
Three pairs of proposed resonance structures are given.
Long Description
(Must be marked up in HTML)
In each pair, the structures only differ in the placement of double and single bonds.
- In the first pair, the structures are hexagonal rings with alternating single and double bonds. In one hexagonal ring, the double bonds begin between C 1 and C 2, and in the other double bonds begin between C 2 and C 3. They are resonance structures.
- In the second pair, the structures are squares with alternating single and double bonds. In one square ring, the double bonds begin between C 1 and C 2, and in the other double bonds begin between C 2 and C 3. They are not resonance structures.
- In the third pair, the structures are octagonal rings with alternating single and double bonds. In one octagonal ring, the double bonds begin between C 1 and C 2, and in the other double bonds begin between C 2 and C 3. They are not resonance structures.
Example 11
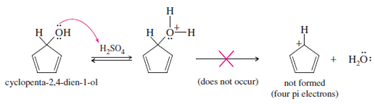
Alt Text
A reaction scheme is given.
Long Description
(Must be marked up in HTML)
Cyclopenta-2,4-dien-1-ol reacts in the presence of H 2 S O 4 to yield a protonated intermediate. The intermediate does not lose water because it would yield an unstable antiaromatic cation with four pi electrons.
- Cyclopenta-2,4-dien-1-ol is a pentagonal ring with a double bond between C 2 and C 3 and between C 4 and C 5. C 1 has a single bond to H and another single bond to O H, where O has two lone pairs. One of the lone pairs forms a bond with H of H 2 S O 4 to yield the protonated intermediate.
- The intermediate is a pentagonal ring with a double bond between C 2 and C 3 and between C 4 and C 5. C 1 has a single bond to H and another single bond to O, which, in turn, is single-bonded to two H atoms. O has a lone pair and carries a positive charge.
- The antiaromatic cation that does not form is a pentagonal ring with a double bond between C 2 and C 3 and between C 4 and C 5. C 1 has a single bond to H and carries a positive charge.
Example 12
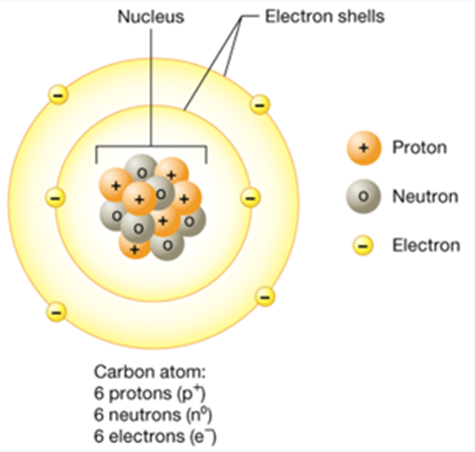
Alt Text
The structure of a representative neutron shows a cluster of protons and neutrons in the center, forming a nucleus.
Long Description
(Must be marked up in HTML)
The nucleus is surrounded by two electron shell rings. The inner ring has two electrons, and the outer ring has four. In a carbon atom, there are 6 protons or p plus, 6 neutrons, or n 0, and 6 electrons, or e minus.
Dated: 2023-12-01
